U.S. and Russian Scientists Make Hydrogen Fuel With Light and Fats

Solar energy steals most of the spotlight these days when it comes to renewable energy. But hydrogen fuel has always remained a promising candidate for a green energy source. Hydrogen never hit its stride like solar did; it never became exponentially easier or cheaper to produce and distribute over time.
But U.S. and Russian scientists may have just figured out to produce hydrogen much more efficiently by utilizing sunlight and lipids (fat). If it can be scaled up, this method may have profound implications for the future of energy.

Pure as Water
When you burn hydrogen in fuel cells, water vapor is the only byproduct. So, it’s no wonder that some consider it the cleanest energy alternative. Hydrogen’s fuel efficiency is also usually around 45 percent or greater. This is astounding compared to gasoline fuel efficiency, which hovers just below 35 percent.
So why has this wonderful energy source remained so elusive compared to its solar and wind counterparts? It comes down to scalability. Complicated, expensive materials and costly power consumption have prevented hydrogen fuel cells from being manufactured on a larger scale. Yes, car makers like Honda, BMW, and Toyota all have hydrogen-powered cars on the market. But they’re not nearly as ubiquitous as electric cars because of high costs to obtain hydrogen.
Researchers have been trying to figure out an alternative to produce hydrogen for quite some time. Utilizing other energy sources has proven to be helpful. For instance, hydrogen can be made from water, solar power, and special compounds, known as photocatalysts.
Titanium dioxide is a popular example of a photocatalyst. It’s not even close to being the most effective one though, so boosting its capabilities has been a staple of hydrogen fuel research in recent years. Grinding it or introducing chemical impurities (known as doping) are common methods used to do this.
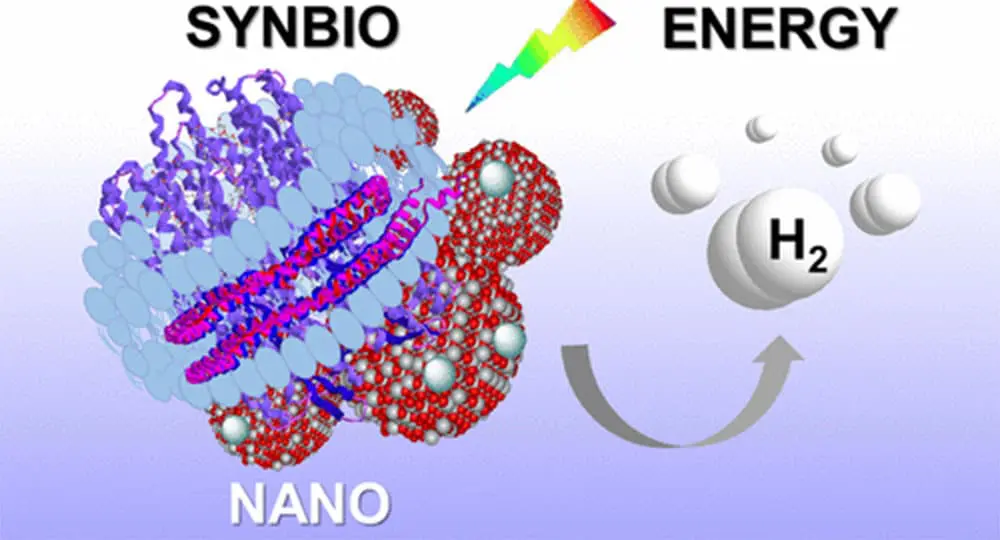
Scientists from Illinois’ Argonne National Laboratory and the Moscow Institute of Physics and Technology (MIPT) have put a new twist on the practice. By using a biological nanostructure made from lipids, these researchers may have just mass hydrogen fuel cell usage viable.
Some Light, Some Fat, and Some Brains
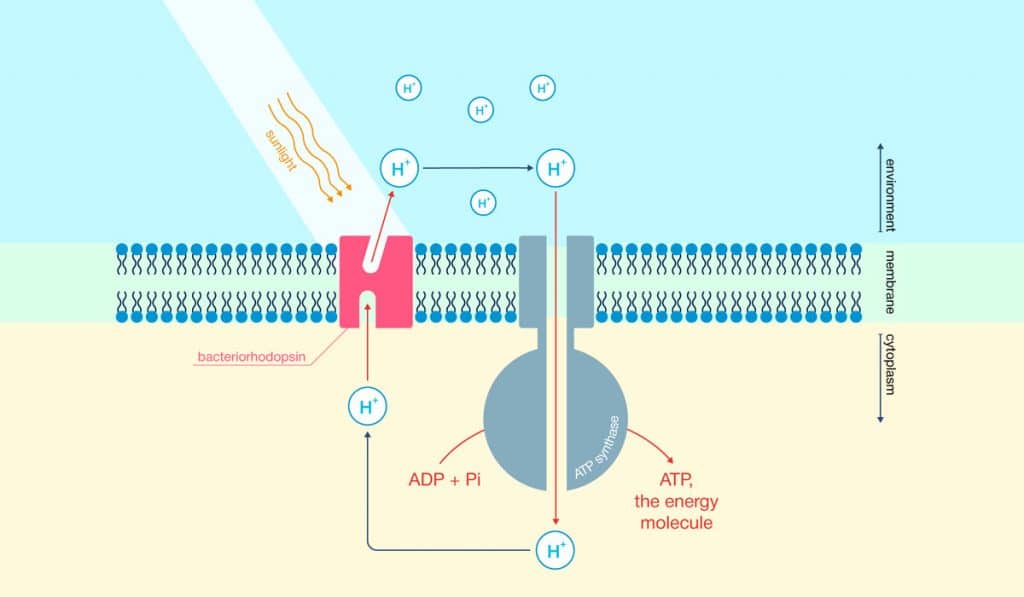
The researchers started things off differently by inserting Bacteriorhodopsin, a photosensitive protein, into nanodiscs. This protein is usually part of microbial cell membranes. The nanodiscs, composed of lipids, essentially acted as this membrane. To actually induce photocatalysis (the acceleration of a chemical reaction with light), the researchers dissolved the nanodiscs in water. They added titanium dioxide as well as platinum, which makes the reaction more potent.
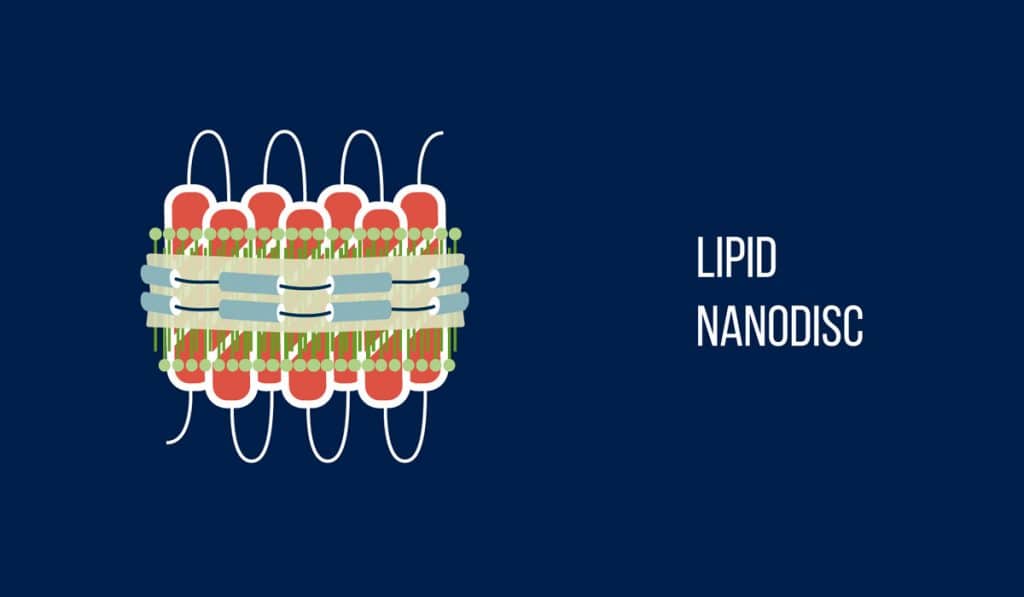
Overnight, the nanodiscs adhered to the catalytic particles introduced to the mix earlier. Bacteriorhodopsin would then act as an energy transmitter by capturing light and giving the energy to the titanium dioxide particles. This increased the overall mechanism’s light sensitivity. Bacteriorhodopsin also acted as a natural proton transporter. When mixed with the platinum catalyst, this resulted in hydrogen. In order to drive up the amount of hydrogen being produced, researchers added methanol (a source of electrons), which effectively reduced the number of protons.
The scientists also experimented with the type of light “fed” to the mixture by exposing one test to green light and the other to white light. White light produced 74 times more hydrogen than green light. On average, hydrogen emission remained constant for about two to three hours.
Big Surprises Come in Small Packages
The scientists themselves were of course more than pleased with the results. Professor Vladimir Chupin, who runs the Laboratory of the Chemistry and Physics of Lipids at MIPT, elaborates on his surprised satisfaction: “Our laboratories working with membrane proteins, in particular with nanodiscs, are mostly focused on biophysical and medical issues. However, the recent joint study with our U.S. colleagues shows that by bringing together biological and technical materials, nanodiscs can be used to obtain hydrogen fuel.”
ACS Nano recently published the scientists’ work. The experiments were all done on a nanoscale level. This makes it difficult to convey the implications of the results on a macro scale.
As the paper puts it, “The system produces hydrogen at a turnover of about 240 μmol of H2 (μmol protein)−1 h–1 and 17.74 mmol of H2 (μmol protein)−1 h–1 under monochromatic green and white light, respectively, at ambient conditions, in water at neutral pH and room temperature… Robustness and flexibility of this approach allow for systemic manipulation at the nanoparticle–bio interface toward directed evolution of energy transformation materials and artificial systems.”
While the scientists remain humble and pragmatic discussion of their work, the importance can’t be understated. Their experiments could be the catalyst to boost hydrogen towards the forefront of clean energy. The Tesla Model S and Model 3 are nice, but there would be nothing wrong with seeing more hydrogen-fueled automobiles on the road as well.
Sources: Phys, Moscow Institute of Physics and Technology, Futurism, ACS Nano, EconoTimes